Press Release
Stockholm, September 18, 2006
DIAMYD MEDICAL ADDS TO PREVIOUSLY REPORTED POSITIVE EFFICACY RESULTS WITH DIAMYD™ IN TYPE 1 DIABETES PATIENTS AT A EUROPEAN SCIENTIFIC DIABETES MEETING IN COPENHAGEN
Press Release, Stockholm, Sweden - September 18, 2006 - Diamyd Medical AB
(SWEDEN OMXS: DIAM B; USA ADR: DMYDY) Updated January 28, 2007
Professor Johnny Ludvigsson, Linkoping University Hospital, Linkoping, Sweden and Principal Investigator for the Diamyd™ phase II study in 70 patients with recent onset type 1-diabetes, presented further results from the study at the European Diabetes meeting EASD in Copenhagen.
Major conclusions were:
· 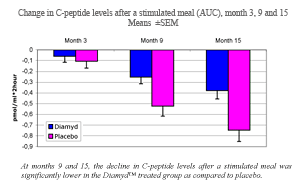 Two single administrations of Diamyd™, four weeks apart, demonstrated safety and efficacy in slowing the decline of C-peptide levels after a stimulated meal at 15 months.
Two single administrations of Diamyd™, four weeks apart, demonstrated safety and efficacy in slowing the decline of C-peptide levels after a stimulated meal at 15 months.
· Diamyd™ treated patients required less insulin than placebo patients.
· Diamyd™ treated patients with a disease duration shorter than 3 months showed an improved insulin production.
· Diamyd™ offers a compelling, first in class, therapeutic for beta cell preservation in type 1-diabetes, in particular due to ease of use and patient acceptance.
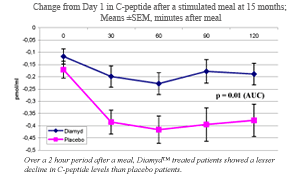 As announced previously, the results from the Diamyd™ study demonstrated that the group of 35 recently diagnosed type 1-diabetes patients that received Diamyd™ experienced on average only half the decline in meal stimulated insulin, as measured by C-peptide levels (Area Under the Curve) 15 months after the first treatment as compared to the placebo group (p=0.01). As insulin and C-peptide are produced in equal amounts and C-peptide is easier to measure, meal stimulated C-peptide levels is the most important parameter to follow in a type 1-diabetes study where the aim is to preserve beta cell function. C-peptide levels in both groups experienced a decline but the decline was significantly inhibited in the Diamyd™ group. There were no significant differences in fasting C-peptide levels between the two groups
As announced previously, the results from the Diamyd™ study demonstrated that the group of 35 recently diagnosed type 1-diabetes patients that received Diamyd™ experienced on average only half the decline in meal stimulated insulin, as measured by C-peptide levels (Area Under the Curve) 15 months after the first treatment as compared to the placebo group (p=0.01). As insulin and C-peptide are produced in equal amounts and C-peptide is easier to measure, meal stimulated C-peptide levels is the most important parameter to follow in a type 1-diabetes study where the aim is to preserve beta cell function. C-peptide levels in both groups experienced a decline but the decline was significantly inhibited in the Diamyd™ group. There were no significant differences in fasting C-peptide levels between the two groups
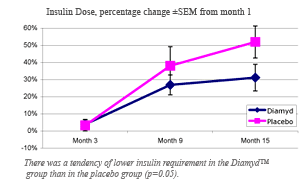 · The relative insulin requirements in the Diamyd™ treated group increased less than in the placebo group compared on a percentage basis (p=0.05).
· The relative insulin requirements in the Diamyd™ treated group increased less than in the placebo group compared on a percentage basis (p=0.05).
· There was no difference in HbA1c levels between the Diamyd™ group and the placebo group. This is consistent with type 1-diabetes patients striving to reach normal blood glucose levels through their standard insulin treatments.
· There was a tendency of increased GAD antibody levels in the Diamyd™ group compared to the placebo group indicating that the drug candidate has an immunomodulating effect.
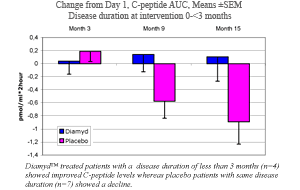
· Although subgroups were too small for statistical calculations, Diamyd™ treated patients with a disease duration of less than 3 months (n=4) showed improved C-peptide levels at 15 months, whereas placebo treated patients (n=7) showed a decline.
These results provide strong support that the administration of Diamyd™ is effective in preserving islet cell function in type 1-diabetes patients. Additionally, maintenance of endogenous insulin production is important as it helps patients to better control their disease and reduce long-term complications.
Finally, as in all previously reported clinical studies with Diamyd™, the results also strongly support the safety of Diamyd™ administration. There were no serious adverse events reported that were related to the Diamyd™ treatment.
The study is now in a follow up stage of 15 months. The Clinical Trial Report from this study is currently in preparation.
"With these positive results in the type 1-diabetes study, the likelihood that the ongoing Phase II Clinical Trial with 160 LADA-patients will be successful has increased", says Anders Essen-Moller, President and CEO of Diamyd Medical. Preliminary results from the LADA trial are planned to be presented in the summer 2007.
About Diamyd Medical:
Diamyd Medical is a Life Science company focused on developing treatments for diabetes and its complications. The Company´s furthest developed project is the GAD-based drug Diamyd™ against autoimmune diabetes. Diamyd™ is currently involved in ongoing clinical Phase II trials of both type 1- and type 2-diabetes patients.
GAD65 is a dominating autoantigen in autoimmune diabetes and is the active substance in Diamyd™. GAD65 is also an enzyme that converts the excitatory neurotransmittor glutamate to the inhibitory transmittor GABA. In this context GAD may have an important role not only in diabetes, but also in several CNS-related diseases. Diamyd Medical has an exclusive world-wide license from the UCLA in Los Angeles regarding the therapeutic use of the GAD65 gene. . It also has been granted a license from the University of Florida for the use of GAD in therapeutic applications related to the treatment of type 1-diabetes.
Diamyd Medical has outlicensed the use of the GAD65-gene to Neurologix Inc., New York, for treatment of Parkinson´s disease and clinical Phase I studies are ongoing.
Other projects comprise drug development within gene therapy using the patent protected NTTDS system (Nerve Targeted Drug Delivery System). The projects mainly make use of Enkephalin and GAD and are targeted for chronic pain e.g. diabetes pain or cancer pain. All projects in this field are in preclinical phases.
Diamyd Medical has operations in Stockholm (Sweden) and in Pittsburgh (USA) and its shares are quoted at the Stockholmborsen O-List (OMX:DIAM B). The Diamyd share is also traded in the US through a Level 1 ADR program administered by the Bank of New York. (ticker symbol: DMYDY). Further information is to be found on the Company´s website; www.diamyd.com.
For further information, please contact:
Anders Essen-Möller, President, Tel: +46 (0) 8-661-0026, Cell: +46-70-551-0679
Diamyd Medical AB (publ). Linnégatan 89 B, SE-115 23 Stockholm, Sweden. Tel: +46 8 661 00 26, fax: +46 8 661 63 68 or email: info@diamyd.com. VATno: SE556530-142001.
This Information may include statements concerning historical, present and forward-looking items and are to the "best of knowledge" of the management of Diamyd Medical and the actual status may differ materially from these statements. The Company assumes no obligation to update these statements to reflect actual results, changes in assumptions or changes in other factors affecting such statements. The Company´s Press Releases, Quarterly Reports and Annual Reports ("Information") are translations from Swedish originals. No guarantees are made that these translations are free from errors.